Track 2: Novel Strategies to Advance Biotherapeutic Development
Category: Poster Abstract
(T1130-02-07) Anti-CCR8 Mediated Depletion of Tumor-Infiltrating Regulatory T Cells: Preclinical PK/PD and Translational Strategy in Support of Clinical Development for Solid Tumor Indications
- GG
Gautham Gampa
Genentech, Inc.
South San Francisco, California, United States - GG
Gautham Gampa
Genentech, Inc.
South San Francisco, California, United States - PS
Phillip Spinosa
Genentech, Inc.
South San Francisco, California, United States 
Jennifer Getz
Senior Principal Scientist
Genentech, Inc.
South San Francisco, California, United States- YZ
Yu Zhong
Genentech, Inc.
South San Francisco, California, United States - WH
Wendy Halpern
Genentech, Inc.
South San Francisco, California, United States - EE
Emel Esen
Genentech, Inc.
South San Francisco, California, United States - JD
John Davies
Genentech, Inc.
South San Francisco, California, United States - AK
Amrita Kamath
Genentech, Inc.
South San Francisco, California, United States - MH
Mahrukh Huseni
Genentech, Inc.
South San Francisco, California, United States - JS
Jill Schartner
Genentech, Inc.
South San Francisco, California, United States - JK
James Koerber
Genentech, Inc.
South San Francisco, California, United States - SR
Sascha Rutz
Genentech, Inc.
South San Francisco, California, United States - IH
Iraj Hosseini
Genentech, Inc.
South San Francisco, California, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Regulatory T (Treg) cells represent a major immunosuppressive cell type in tumors 1. The presence of Treg cells is associated with poor clinical outcomes in cancer patients 2, 3. Thus, depletion of Treg cells in tumors represents a promising therapeutic approach to reverse immunosuppression. C-C motif chemokine receptor 8 (CCR8) is a unique target with selective expression on activated and proliferating intratumoral Treg cells 4,5. RO7502175 is an afucosylated antibody designed to eliminate CCR8 expressing Treg cells in the tumor microenvironment through enhanced antibody-dependent cellular cytotoxicity (ADCC). Herein, we describe the translational pharmacokinetic (PK)/pharmacodynamic (PD) approach used to inform first-in-human (FiH) dosing strategy and clinical development of RO7502175 in solid tumor indications.
Methods: A systematic preclinical assessment comprising both in vitro and in vivo (mouse and cynomolgus monkey) studies was performed to characterize the PK/PD and safety profile of anti-CCR8 antibodies. A minimal physiologically-based PK-PD (mPBPK-PD) model was developed to support clinical translation and enable projection of clinical PK and receptor occupancy (RO) for RO7502175 in patients. The mechanistic model incorporated explicit representation of anti-CCR8 antibody PK, antibody binding to CCR8 receptors, CCR8+ Treg cell depletion, CD8+ T cell increases, and tumor cell killing. The selection of FiH dose used an integrated approach that was based on the totality of preclinical data and was supported by modeling insights.
Results: RO7502175 exhibited selective ADCC against human CCR8 expressing Treg cells in in vitro assays. A surrogate anti-murine CCR8 antibody demonstrated efficient PD activity and anti-tumor efficacy in syngeneic mouse tumor models, establishing proof-of-concept. In cynomolgus monkeys, RO7502175 demonstrated dose-proportional increase in systemic exposures, reduced CCR8 expressing Treg cells in blood (PD readout), and caused minimal and transient cytokine secretion (Fig. 1). No RO7502175-related toxicity findings were observed in cynomolgus monkey studies and 100 mg/kg dose was identified as the no observed adverse effect level (NOAEL). Moreover, RO7502175 caused minimal cytokine release from PBMCs in in vitro assays. To explain the PK-PD-efficacy relationship, a quantitative modeling framework was developed for capturing surrogate anti-murine CCR8 antibody PK/PD and tumor profiles in mice, and RO7502175 PK profiles in cynomolgus monkeys (Fig. 2). The model was then used to project RO7502175 clinical PK and RO in patients (Fig. 3). The proposed FiH dose of 2 mg IV for RO7502175 was based on an integrated approach utilizing the comprehensive nonclinical data and was guided by insights from mPBPK-PD model. RO7502175 was well-tolerated with a NOAEL of 100 mg/kg in cynomolgus monkey studies, and this provides a high safety margin of greater than 3000-fold for the proposed FiH dose in humans. The proposed starting dose for the Phase I clinical study is expected to be safe in the clinic, provide some level of pharmacological activity and decrease administration of sub-therapeutic dose levels to patients.
Conclusion: The research findings and work described represent a translational strategy for gathering and utilizing appropriate preclinical data, developing a mechanistic PK/PD model framework, and using an integrated approach to inform the study design for testing RO7502175 in patients. Since traditional approaches such as MABEL-based and mPAD approaches would have resulted in a rather low clinical starting dose for RO7502175, even with its superior nonclinical safety profile, an integrated approach based on the comprehensive preclinical data and model informed insights was used for the selection of FiH dose. Overall, the nonclinical PKPD data package supports the Phase I clinical evaluation of RO7502175 in solid tumor indications.
References: 1. Wing, J. B., Tanaka, A., & Sakaguchi, S. (2019). Human FOXP3+ Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity, 50(2), 302–316.
2. Peng, L.S., Zhuang, Y., Shi, Y., Zhao, Y.L., Wang, T.T., Chen, N., Cheng, P., Liu, T., Liu, X.F., Zhang, J.Y., Zuo, Q.F., Mao, X.H., Guo, G., Lu, D.S., Yu, P.W., & Zou, Q.M. (2012). Increased tumor-infiltrating CD8(+)Foxp3(+) T lymphocytes are associated with tumor progression in human gastric cancer. Cancer Immunology, Immunotherapy: CII, 61(11), 2183–2192.
3. Togashi, Y., Shitara, K., & Nishikawa, H. (2019). Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nature Reviews. Clinical Oncology, 16(6), 356–371.
4. De Simone, M., Arrigoni, A., Rossetti, G., Gruarin, P., Ranzani, V., Politano, C., Bonnal, R. J. P., Provasi, E., Sarnicola, M. L., Panzeri, I., Moro, M., Crosti, M., Mazzara, S., Vaira, V., Bosari, S., Palleschi, A., Santambrogio, L., Bovo, G., Zucchini, N., … Pagani, M. (2016). Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity, 45(5), 1135–1147.
5. Zheng, C., Zheng, L., Yoo, J.K., Guo, H., Zhang, Y., Guo, X., Kang, B., Hu, R., Huang, J. Y., Zhang, Q., Liu, Z., Dong, M., Hu, X., Ouyang, W., Peng, J., & Zhang, Z. (2017). Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell, 169(7), 1342-1356.e16.
Acknowledgements: All authors are current employees of Genentech, Inc. and may be stockholders of F. Hoffmann-La Roche AG. In addition to the listed authors, Vittal Shivva (Senior Principal Scientist at Genentech) has contributed to the work discussed in this abstract.
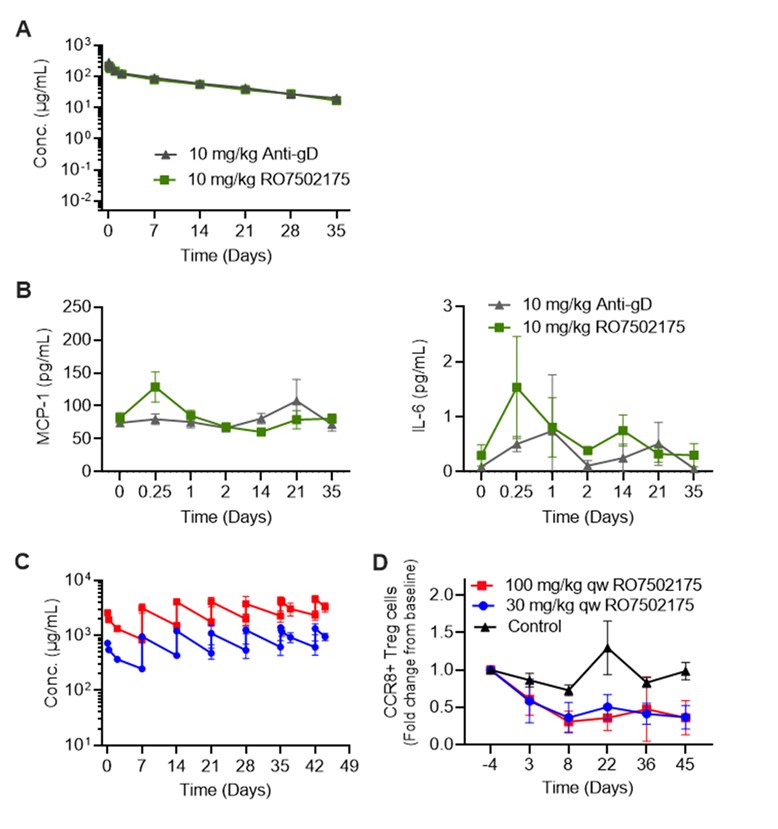 Figure 1. RO7502175 PK, PD and safety profiles in cynomolgus monkeys. (A) Serum concentration-time profiles, and (B) plasma MCP-1 and IL-6 cytokine concentrations following single IV dose of 10 mg/kg anti-gD (control) or RO7502175. (C) Serum concentration-time profiles, and (D) fold change from baseline of CCR8+ Treg cells in blood following IV dosing of vehicle control or RO7502175 in a repeat-dose study.
Figure 1. RO7502175 PK, PD and safety profiles in cynomolgus monkeys. (A) Serum concentration-time profiles, and (B) plasma MCP-1 and IL-6 cytokine concentrations following single IV dose of 10 mg/kg anti-gD (control) or RO7502175. (C) Serum concentration-time profiles, and (D) fold change from baseline of CCR8+ Treg cells in blood following IV dosing of vehicle control or RO7502175 in a repeat-dose study.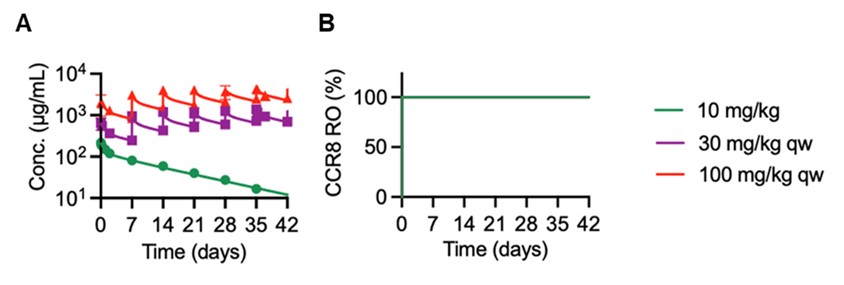 Figure 2. mPBPK-PD model (A) captures RO7502175 PK profiles in cynomolgus monkeys, and (B) predicted RO profiles at the respective dose levels are shown.
Figure 2. mPBPK-PD model (A) captures RO7502175 PK profiles in cynomolgus monkeys, and (B) predicted RO profiles at the respective dose levels are shown.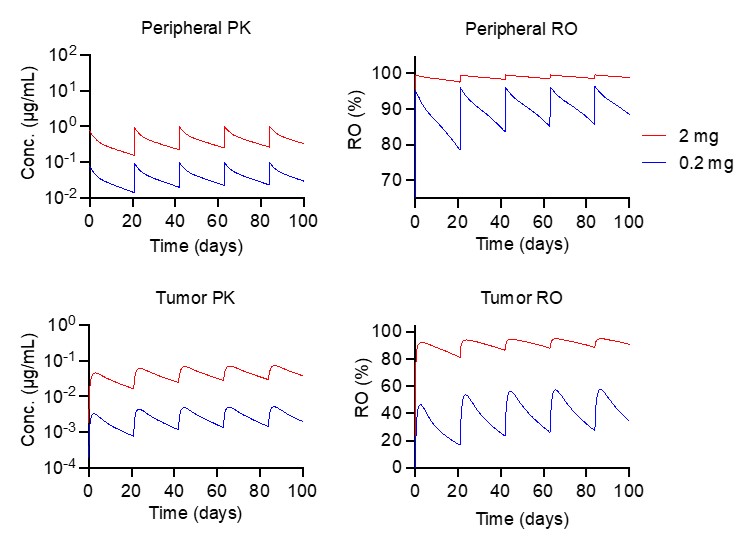 Figure 3. Projected RO7502175 clinical PK and receptor occupancy (RO) in plasma and tumor in patients.
Figure 3. Projected RO7502175 clinical PK and receptor occupancy (RO) in plasma and tumor in patients.