Track 1: Advances in Discovery, Formulation, and Delivery of New Modalities
Category: Poster Abstract
(W1430-01-02) High Concentration Protein Suspensions: A Case Study for Subcutaneous Delivery of Bevacizumab
Wednesday, May 15, 2024
2:30 PM - 3:30 PM PT
- ES
Erica Schlesinger
Seran Biosciences
Bend, Oregon, United States - ES
Erica Schlesinger
Seran Biosciences
Bend, Oregon, United States
Presenting Author(s)
Main Author(s)
Purpose: There is a growing interest across the industry in developing technologies for subcutaneous delivery of high dose protein therapeutics. High concentration protein solutions are limited in their application to this space by viscosity and stability; both phenomena are driven by protein-protein interactions in solution. High concentration suspensions have the potential to address both the syringability and stability challenges as well as to provide a broadly applicable technology platform. Spray drying is one technique available for the production of protein microparticles suitable for incorporation into suspension. This case study presents the development of a high concentration protein suspension (400-500 mg/mL) suitable for subcutaneous delivery of monoclonal antibody (mAb) and considers both protein stability and suspension viscosity.
Methods: Protein microparticles were produced via spray drying and suspended in non-aqueous vehicles. Particles were engineered and vehicles selected to minimize viscosity for delivery of the highest concentrations possible through a PFS with a 27g ½ in needle. Protein stability in the spray drying process, in the spray dried intermediate, and in the suspension was evaluated using size exclusion chromatography. The intermediate and suspensions were evaluated under open-dish accelerated conditions to determine the relative effect of temperature and moisture on protein stability.
Results: The mAb remained stable during the spray drying process, with no evidence of increasing HMW in the spray solution, collection vessel, or before and after the atomization and drying process (Figure 1). While the spray dried protein (SDP) on its own showed evidence of aggregation when exposed to high humidity, incorporation of the SDP into the non-aqueous vehicle conferred protection against moisture and demonstrated improved stability (Figure 2); long-term stability studies are on-going. Both the SDP and SDP in suspension demonstrate improved stability over the current solution formulation for IV administration. In prior work, the relationship between spray dried powder density and syringe dispensing force was established for a range of model compounds. SDP comprised of mAb showed similar behavior (Figure 3) and demonstrates the impact of particle engineering on product optimization. Furthermore, the data in Figure 3 illustrates that a suspension containing 40wt% mAb SDP can be administered through a 27g ½ inch needle with less than 20N glide force.
Conclusion: The feasibility of a high concentration suspension comprised of a spray dried mAb in a non-aqueous vehicle to deliver a 40 wt% suspension was demonstrated. Additional results suggest that up to 60wt% mAb SDP can be administered with less than 50N glide force in an optimized vehicle. Protein stability as measured by SEC was demonstrated in the spray drying process and for the protein in suspension. Additional studies are on-going to evaluate the impact of formulation excipients in the SDP on protein stability and suspension behavior, to evaluate stability on longer time-scales and with additional analytical methods, and to evaluate the impact of vehicle composition on suspension behavior and stability.
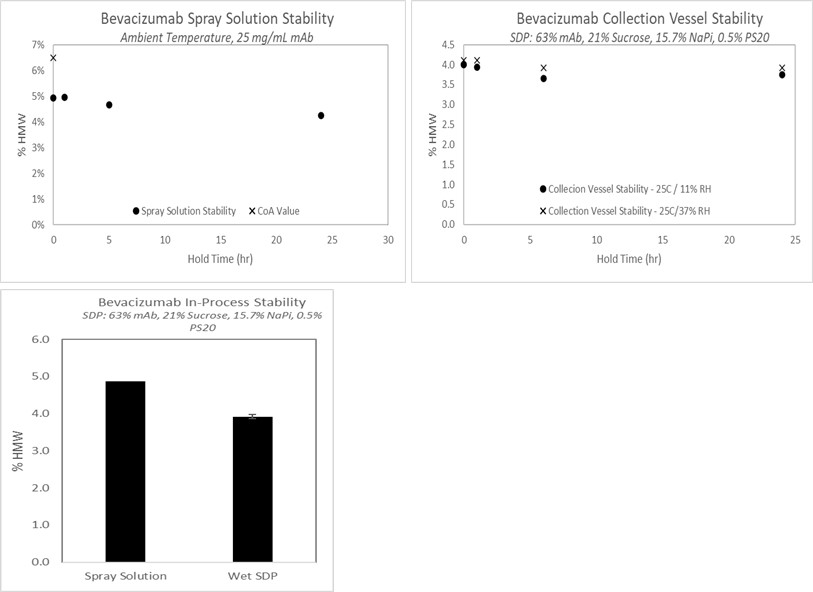 Figure 1
Figure 1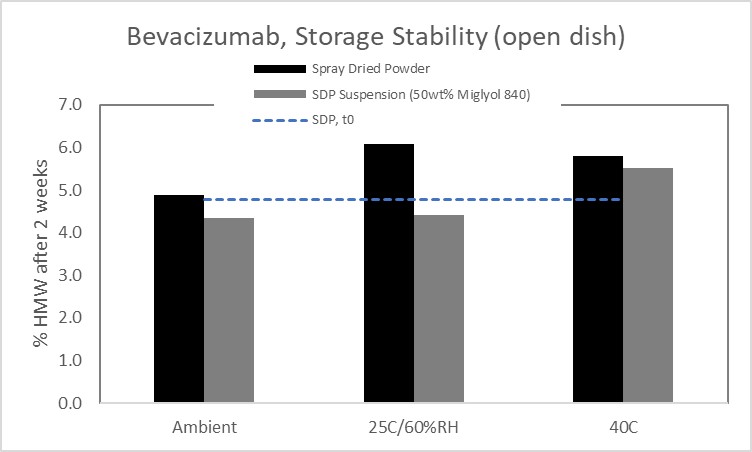 Figure 2
Figure 2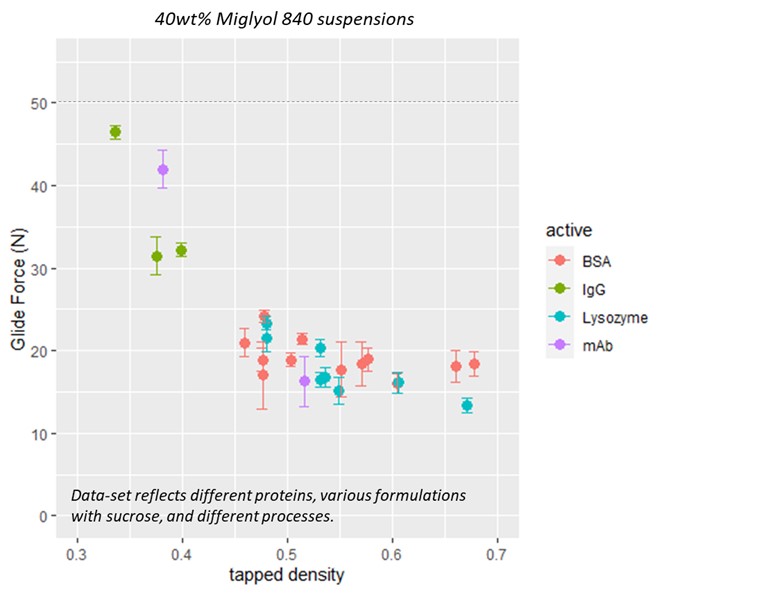 Figure 3
Figure 3