Track 1: Advances in Discovery, Formulation, and Delivery of New Modalities
Category: Poster Abstract
(T1230-01-01) Parallel-Competitive Absorption Metabolism Model for Calculation of Subcutaneous Bioavailability of Monoclonal Antibodies
Tuesday, May 14, 2024
12:30 PM - 1:30 PM PT

Mikolaj Milewski, PhD
Principal Scientist
Merck & Co., Inc.
Lansdale, Pennsylvania, United States
Mikolaj Milewski, PhD
Principal Scientist
Merck & Co., Inc.
Lansdale, Pennsylvania, United States
Mikhail Murashov
Assoc Prin. Scientist
Merck & Co., Inc.
Rahway, New Jersey, United States- YK
Yash Kapoor
Merck & Co., Inc.
West Point, PA 19486, Pennsylvania, United States - MC
Maria Cueto
Merck & Co., Inc.
Rahway, New Jersey, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Scientists developing new therapeutic monoclonal antibodies (mAbs) for subcutaneous (SC) administration face a significant challenge. This challenge is the absence of a dependable predictive method for determining the clinical bioavailability of biologics. The level of systemic absorption directly affects the selection of subcutaneous doses and impacts the cost of goods during drug product development. The main aim of the current research was to thoroughly assess the subcutaneous bioavailability data of commercially approved drug products and develop a new method for estimating bioavailability of monoclonal antibodies.
Methods: The PK dataset of 19 mAbs with a reported average bioavailability of 68% was adapted directly from Haraya et al. publication (Drug Metabolism and Pharmacokinetics 32 (2017) 208-217). Authors used multiple sources including scientific literature, patents, presentations at conferences, information from the Pharmaceutical and Medical Devices Agency (PMDA) and FDA, and their own pharmacokinetic modeling in assembling this dataset. A validation dataset with an average bioavailability of 69% was independently compiled using published sources for 5 commercial drug products. Subsequently, a novel parallel-competitive absorption and presystemic metabolism model was applied to calculate SC bioavailability from IV parameters alone.
Results: The SC bioavailability of FDA-approved therapeutic mAbs typically falls within the range of 60-80%. Notable extreme values are exemplified by 53% (observed in golimumab, Simponi®, through cross-trial comparisons of mean AUCinf values) and 96% (observed in tocilizumab, Actemra® in polyarticular juvenile idiopathic arthritis patients). In an analysis conducted by Haraya et al., it was observed that there exists an inverse correlation between subcutaneous bioavailability and systemic clearance of mAbs in humans. The findings of that study suggested that the presystemic clearance of linear-pharmacokinetic mAbs was directly proportional to their systemic clearance. Here, we built on this observation by postulating that presystemic metabolism rate constant can be estimated directly from the elimination rate constant of the central compartment of a 2-compartment PK model. First, a single absorption rate constant and individual estimates of presystemic metabolism rate were utilized to accurately calculate the subcutaneous bioavailability of these antibodies in humans. The calculated bioavailability closely resembled the clinical data in the training dataset, with a mean root-square error of 5.5%. Then, when the same approach was applied to the validation dataset, the predictions resulted in a mean root-square error of 12.6%.
Conclusion: An ability to predict the SC bioavailability of mAbs prior to first subcutaneous dosing in the clinic could improve the efficiency of the drug product development process and aid in the selection of appropriate doses for clinical studies. Published models for predicting SC bioavailability focus on either mAb physicochemical properties / formulation attributes or the physiology / pharmacokinetics and are empirical in nature. None of the existing approaches reported in the literature incorporates both aspects within a single model framework. In the new model that presented here, we explicitly captured the roles of absorption and presystemic metabolism in a mechanistic manner and demonstrated the prediction accuracy of the novel model in the training and validation data sets. Future work based on the approach reported here could include studying effects related to systemic clearance changes (e.g. effects of mAb bioengineering) or absorption rate changes (e.g. site of injection effects or co-administration with hyaluronidase) on clinical bioavailability.
References: Haraya et al. Quantitative prediction of therapeutic antibody pharmacokinetics after intravenous and subcutaneous injection in human Drug Metab Pharmacokinet. 2017 Aug; 32(4):208-217. doi: 10.1016/j.dmpk.2017.05.002.
Acknowledgements: Conflict of interest: The authors report no conflict of interest.
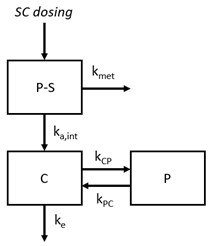 Figure 1. A schematic of the parallel-competitive absorption presystemic metabolism model including a presystemic (P-S) absorption compartment and two systemic compartments: central (C) and peripheral (P) (aka a 2-compartment model)
Figure 1. A schematic of the parallel-competitive absorption presystemic metabolism model including a presystemic (P-S) absorption compartment and two systemic compartments: central (C) and peripheral (P) (aka a 2-compartment model)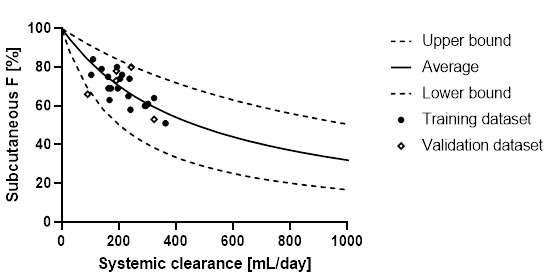 Figure 2. The SC bioavailability as a function of systemic clearance. Individual data points are experimental subcutaneous bioavailability values from a training dataset (filled circles) and a validation dataset (open diamonds). Lines correspond to the calculated subcutaneous bioavailability. The solid line is the mean prediction value while low- and high-limit dashed lines corresponds to the prediction space limited by the range of clinical ka,int values
Figure 2. The SC bioavailability as a function of systemic clearance. Individual data points are experimental subcutaneous bioavailability values from a training dataset (filled circles) and a validation dataset (open diamonds). Lines correspond to the calculated subcutaneous bioavailability. The solid line is the mean prediction value while low- and high-limit dashed lines corresponds to the prediction space limited by the range of clinical ka,int values 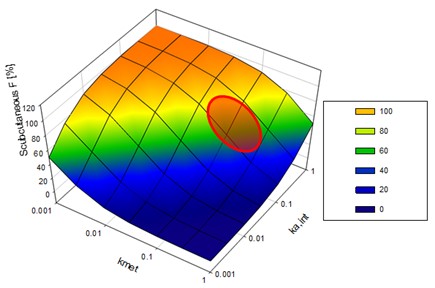 Figure 3. Parameter sensitivity analysis in ka,int and kmet in prediction of the subcutaneous bioavailability of mAbs according to the proposed model. The highlighted region corresponds to approximate ranges of calculated values of ka,int and kmet.
Figure 3. Parameter sensitivity analysis in ka,int and kmet in prediction of the subcutaneous bioavailability of mAbs according to the proposed model. The highlighted region corresponds to approximate ranges of calculated values of ka,int and kmet.