Track 2: Novel Strategies to Advance Biotherapeutic Development
Category: Poster Abstract
(T1230-02-09) Population Pharmacokinetics and Exposure-Efficacy and -Safety Analyses of Atezolizumab for Subcutaneous Injection in Patients with Locally Advanced or Metastatic Non-small Cell Lung Cancer

Phyllis Chan, PhD (she/her/hers)
Distinguished Scientist
Genentech, Inc.
South San Francisco, California, United States
Phyllis Chan, PhD (she/her/hers)
Distinguished Scientist
Genentech, Inc.
South San Francisco, California, United States- SL
Stephanie Liu
Genentech, Inc.
South San Francisco, California, United States - NG
Nathalie Gosselin
Certara
Montréal, Quebec, Canada - ZS
Zacharie Sauve
Certara
Montréal, Quebec, Canada - MM
Mathilde Marchand
Certara
Paris, Ile-de-France, France - AL
Alyse Lin
Genentech, Inc.
South San Francisco, California, United States - LH
Luis Herraez-Baranda
F. Hoffmann-La Roche Ltd.
Basel, Schwyz, Switzerland - JZ
James Zanghi
Genentech, Inc.
South San Francisco, California, United States - ES
Esther Shearer-Kang
Genentech, Inc.
South San Francisco, California, United States - XL
Xiaoyan Liu
Genentech, Inc.
South San Francisco, California, United States - BW
Benjamin Wu
Genentech, Inc.
South San Francisco, California, United States - PC
Pascal Chanu
Genentech, Inc.
South San Francisco, California, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Atezolizumab, an immunoglobulin G1 monoclonal antibody that targets human programmed death‐ligand 1 (PD‐L1), is approved for intravenous (IV) administration to treat various solid tumor indications by the US Food and Drug Administration and the European Medicines Agency [1, 2]. IMscin001 is a two-part dose-finding (Part 1) and confirmation (Part 2) study to evaluate atezolizumab pharmacokinetics of subcutaneous (SC) compared to IV administration in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) [3, 4]. The objectives of these analyses were to characterize the population pharmacokinetics (popPK) of SC atezolizumab, and to determine the relationship between popPK model-predicted atezolizumab exposure metrics and clinical endpoints in the IMscin001 study.
Methods: The popPK analysis was performed using data from all randomized patients in IMscin001 with at least one reportable atezolizumab administration and a corresponding post-dose measurable PK concentration. The atezolizumab IV popPK model [5] was extended to add SC absorption parameters, and potential covariates were investigated on the SC absorption parameters. A nonlinear mixed effect modeling approach was used with the FOCEI estimation in NONMEM 7, version 7.3 (ICON, Maryland). Individual estimates of patient-level random effects for rate of absorption (KA) and bioavailability (F1) were plotted vs. a series of baseline covariates to assess any additional covariate effects on the KA or F1 parameters. A prediction-corrected visual predictive check (pcVPC) was performed based on 500 replicates of the popPK model simulation for model qualification. The extended popPK model was used to derive individual exposure metrics at Cycle 1 and steady-state (Cmax, Ctrough, and AUC0-21d) based on individual concentration-time profiles of atezolizumab simulated from the individual empirical Bayes estimates using the actual dosing regimen. Steady-state exposure was assessed after 10 cycles. Impact of baseline covariates on exposure levels of atezolizumab was explored based on descriptive statistics of exposure metrics (AUC0-21d, AUCss, Ctrough Cycle 1, Ctrough,ss, Cmax, Cycle 1 and Cmax,ss). The exposure-response (ER) analysis was performed using data from patients in the SC treatment arm of Part 2 of IMscin001 who received 1875 mg of atezolizumab SC every three weeks (Q3W). The clinical endpoints tested in the analysis were objective response rate (ORR), progression-free survival (PFS), or overall survival (OS) for efficacy and serious adverse events (SAE), adverse events of special interest (AESI), adverse events of Grade 3 to 5 (AEG35), infusion-related reaction (IRR), or injection site reactions (ISR) for safety. Binary safety endpoints, (i.e., ORR and frequency of safety events) were explored by logistic regression versus Cycle 1 exposure as a continuous variable. Time-to-event efficacy endpoints (i.e., PFS and OS) were first explored using Kaplan-Meier estimation stratified by quartiles of Cycle 1 exposure metrics, then by using Cox regression with exposure metrics as continuous variables. The impact of each baseline covariate was explored by integrating it one by one in the logistic or Cox model with the most statistically significant exposure metric. The full covariate model included the most significant exposure metric and all of the statistically significant baseline covariates. Further simplification of the model was performed by removing the non-significant baseline covariates from the full model.
Results: The popPK analysis was conducted using data from 435 patients with 3100 atezolizumab serum samples (average of 7.1 samples/patient). Atezolizumab SC absorption was characterized by a first-order absorption with a bioavailability (F1) of 71.8% and an absorption rate constant (KA) of 0.304 per day. Hemoglobin and albumin were identified as significant covariates on the absorption parameters (F1 and KA, respectively). None of the covariate effects were deemed clinically meaningful, inducing less than a 30% change from the typical value (Figure 1). The final population PK parameter estimates are presented in Table 1. Model validation (i.e., goodness-of-fit, pcVPC plots) suggested that the model adequately described the data. The summary of the individual exposure metrics at Cycle 1 and at steady-state is presented in Table 2 for Part 2 of IMscin001. The exposures following SC and IV administration were generally comparable. The ER analysis dataset comprised of 246 atezolizumab SC-treated patients with exposure data. For all efficacy and safety endpoints, the atezolizumab exposure metric (Cycle 1 AUC0-21d, Ctrough, or Cmax) was not statistically significant (p-value cutoff of 0.05) in the ER models. Even after including all the statistically significant baseline covariates, the most statistically significant atezolizumab exposure metric was still not statistically significant in any of the final ER models.
Conclusion: The extended popPK model was adequate to predict atezolizumab PK after IV and SC administrations in patients with NSCLC and to predict individual exposure metrics. No statistically significant exposure-response relationships were identified with efficacy or safety in patients treated with atezolizumab administered SC at 1875 mg Q3W.
References: 1. European Medicines Agency Tecentriq: EPAR‐product information. https://www.ema.europa.eu/en/documents/product-information/tecentriq-epar-product-information_en.pdf. Accessed October 13, 2023.
2. Food and Drug Administration. Tecentriq: FDA full prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761034s049s051lbl.pdf. Accessed October 13, 2023.
3. Felip E, Burotto M, Zvirbule Z, Herraez-Baranda LA, Chanu P, Kshirsagar S, Maiya V, Chan P, Pozzi E, Marchand M, Monchalin M, Tanaka K, Tosti N, Wang B, Restuccia E. Results of a Dose-Finding Phase 1b Study of Subcutaneous Atezolizumab in Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer. Clin Pharmacol Drug Dev. 2021 Oct;10(10):1142-1155.
4. Burotto M, Zvirbule Z, Mochalova A, Runglodvatana Y, Herraez-Baranda L, Liu SN, Chan P, Shearer-Kang E, Liu X, Tosti N, Zanghi JA, Leutgeb B, Felip E. IMscin001 Part 2: a randomised phase III, open-label, multicentre study examining the pharmacokinetics, efficacy, immunogenicity, and safety of atezolizumab subcutaneous versus intravenous administration in previously treated locally advanced or metastatic non-small-cell lung cancer and pharmacokinetics comparison with other approved indications. Ann Oncol. 2023 Aug;34(8):693-702.
5. Stroh M, Winter H, Marchand M, Claret L, Eppler S, Ruppel J, Abidoye O, Teng SL, Lin WT, Dayog S, Bruno R, Jin J, Girish S. Clinical Pharmacokinetics and Pharmacodynamics of Atezolizumab in Metastatic Urothelial Carcinoma. Clin Pharmacol Ther. 2017 Aug;102(2):305-312.
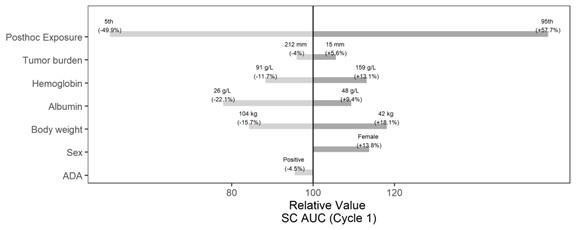 Figure 1: Tornado Plots - Covariate Effects on AUC0-21d (Cycle 1) after Subcutaneous Administration of Atezolizumab SC 1875 mg Q3W. ADA=anti-drug antibodies; AUC0-21d=area under the concentration-time curve during dosing interval at Cycle 1; Q3W=every 3 weeks; SC=subcutaneous.
Figure 1: Tornado Plots - Covariate Effects on AUC0-21d (Cycle 1) after Subcutaneous Administration of Atezolizumab SC 1875 mg Q3W. ADA=anti-drug antibodies; AUC0-21d=area under the concentration-time curve during dosing interval at Cycle 1; Q3W=every 3 weeks; SC=subcutaneous.Note: 5th and 95th percentile of tumor burden, albumin, hemoglobin and body weight in the PK population were retained to evaluate the impact.
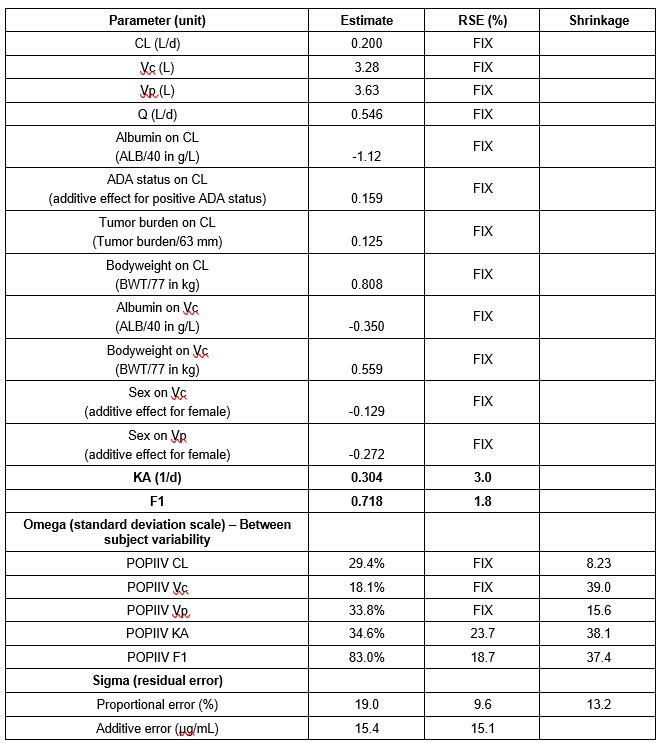 Table 1: Parameter Estimates of the Final popPK Model with Atezolizumab SC and IV Administration for IMscin001. ADA=anti-drug antibodies, CL=clearance from the central compartment, F1=bioavailability, KA=absorption rate constant, POPIIV=inter-individual variability, POPIIV=population inter-individual variability, Q=distributional clearance, RSE=relative standard error, SC=subcutaneous; Vc=volume of distribution of the central compartment, Vp=volume of distribution of the peripheral compartment.
Table 1: Parameter Estimates of the Final popPK Model with Atezolizumab SC and IV Administration for IMscin001. ADA=anti-drug antibodies, CL=clearance from the central compartment, F1=bioavailability, KA=absorption rate constant, POPIIV=inter-individual variability, POPIIV=population inter-individual variability, Q=distributional clearance, RSE=relative standard error, SC=subcutaneous; Vc=volume of distribution of the central compartment, Vp=volume of distribution of the peripheral compartment.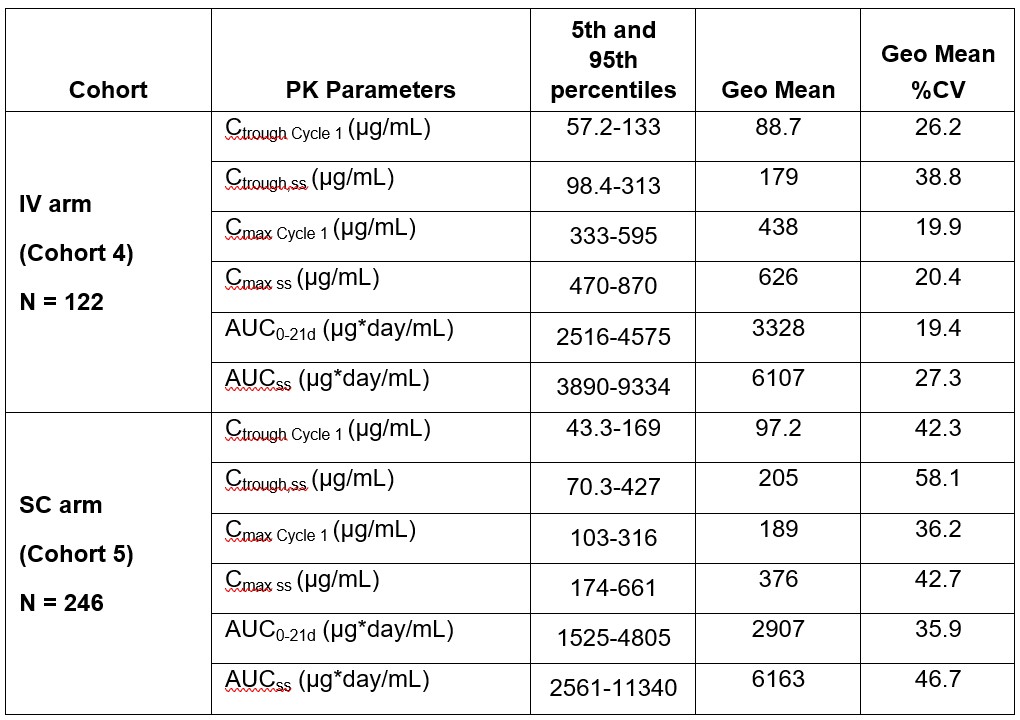 Table 2: Summary Statistics of Atezolizumab Exposure Metrics at Cycle 1 and Steady-State in IMscin001 Part 2 Predicted Using the popPK Model. N=Number of patients; Ctrough,Cycle 1=minimum serum concentration at Cycle 1; Ctrough,ss=minimum serum concentration at steady-state; Cmax Cycle 1=maximum serum concentration at Cycle 1; Cmax ss=maximum serum concentration at steady-state; AUC0-21d=area under the concentration-time curve during dosing interval at Cycle 1; AUCss=area under the concentration-time curve during dosing interval at steady-state, 0-21 days; Geo Mean=geometric mean; CV%=coefficient of variation.
Table 2: Summary Statistics of Atezolizumab Exposure Metrics at Cycle 1 and Steady-State in IMscin001 Part 2 Predicted Using the popPK Model. N=Number of patients; Ctrough,Cycle 1=minimum serum concentration at Cycle 1; Ctrough,ss=minimum serum concentration at steady-state; Cmax Cycle 1=maximum serum concentration at Cycle 1; Cmax ss=maximum serum concentration at steady-state; AUC0-21d=area under the concentration-time curve during dosing interval at Cycle 1; AUCss=area under the concentration-time curve during dosing interval at steady-state, 0-21 days; Geo Mean=geometric mean; CV%=coefficient of variation.