Track 2: Novel Strategies to Advance Biotherapeutic Development
Category: Poster Abstract
(T1430-02-10) A Novel In Vitro Serum Stability Assay for Antibody Therapeutics Incorporating Internal Standards
Tuesday, May 14, 2024
2:30 PM - 3:30 PM PT
- YL
Yihan Li
Abbvie
South San Francisco, California, United States - HS
Hetal Sarvaiya
Abbvie
South San Francisco, California, United States - RV
Rosendo Villafeurte-Vega
University of Michigan
Ann Arbor, Michigan, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: In vitro stability assessment plays a pivotal role in proactively identifying potential liabilities of antibody therapeutics prior to animal studies. The liquid chromatography-mass spectrometry (LC-MS)-based assays typically involve 3 steps: incubation of antibodies in biological matrices, affinity purification, and LC-MS analysis. To our best knowledge, there are no reported instances of routine in vitro stability screening methods involving internal standards (IS) which could be applied to antibody therapeutics across different biological modalities. Uncompensated variations in sample evaporation/precipitation, recovery of affinity purification, and matrix effect during LC-MS analysis could lead to inaccurate stability quantitation, not to mention random operational errors which are difficult to detect and prevent. By incorporating IS, a more accurate stability assessment could be implemented for routine screening.
Methods: Two IS, NISTmAb, a recombinant humanized IgG1ĸ, and Fc fragment of NISTmAb were established in our in vitro serum stability method. Fc fragment of NISTmAb was produced in house by IgdE digestion of NISTmAb, followed by protein A purification. The stability of 19 monoclonal or bispecific antibodies, either in clinical use or clinical trials, were incubated with IS for 7 days in serum of preclinical species such as mouse, rat and cynomolgus monkey. All the in vitro samples were affinity purified using Bravo AssayMAP platform (Agilent Technologies, Santa Clara, CA) with goat anti-human IgG. Triplicate injections of each purified sample were performed on a PLRP-S column (1000 Å, 2.1 × 50 mm, 5 μm) in 1290 Infinity II LC system coupled to 6545xt qTOF mass spectrometer (Agilent Technologies, Santa Clara, CA). Deconvolution of mass spectra and integration of mass peak area of deconvoluted masses were performed in Byos, version 4.5 (Protein Metrics, Cupertino, CA). The mass peak area ratio of antibody and internal standard was calculated, then percent (%) recoveries were calculated by normalizing the mass peak area ratios of samples collected on Day 1, Day 4 and Day 7 to samples collected on Day 0. With the absence of aggregation or biotransformation in the case of stable molecules, the interpretation of stability outcomes would rely on monitoring %recoveries of intact antibodies across the incubation time.
Results: When incubated alone in serum of mouse, rat and cynomolgus monkey, both NISTmAb and Fc fragment exhibited excellent stability across 7-day time, with average %recovery at each time point within 90%-110% range. The %recoveries of two bispecific antibodies (CTX-009 and Glofitamab) with and without NISTmAb as IS were compared and illustrated in Fig 1 as examples. In both cases, the ranges of observed %recovery were improved from 75%-125% (red) to 90%-110% (grey) by incorporating IS. We noticed that NISTmAb could not be separated from antibodies that are similar in size on either the chromatography or mass spectrometry levels, presenting a challenge during data analysis. As a result, 19 antibody therapeutics were divided into two groups for stability assessment in mouse serum: antibodies with molecular weights < 140 kDa (Erfonrilimab, Tidutamab, INBRX-105, Zanidatamab, Tarlatamab, and TNB-738) and >160 kDa (Glofitamab, CTX-009 and Tibulizumab) were incubated with NISTmAb; while Erfonrilimab ( < 140 kDa), Glofitamab ( >160 kDa) and antibodies with molecular weights between 140 and 160 kDa (Bevacizumab, Dupilumab, Palivizumab, Faricimab, Ixekizumab, Evolocumab, Denosumab, Emicizumab, Amivantamab, and Teclistamab) were incubated with Fc fragment. The average %recovery at each time point fell in 90%-110% range for all. Experiments were repeated in rat and cynomolgus monkey serum in the case of Tibulizumab, Erfonrilimab, Bevacizumab, and Dupilumab, with consistent %recovery observed. This demonstrated that both NISTmAb and Fc fragment could serve as IS and improve accuracy of analysis, enabling routine stability assessment by tracking %recovery of intact antibody in serum with more confident acceptance criteria to identify stable molecules (90%-110% instead of 75%-125%). Erfonrilimab ( < 140 kDa) and Glofitamab ( >160 kDa) showed very similar %recovery profiles when analyzed with both NISTmAb and Fc fragment in mouse serum. This indicated that Fc fragment could be a universal IS for antibody therapeutics >60 kDa.
Conclusion: With 19 stable antibodies, we demonstrated that the accuracy of %recovery calculation was improved by incorporating IS for in vitro serum stability assessment. This enabled confident stability assessment by monitoring the trend of %recovery of intact antibodies in serum, in the absence of evidence of aggregation or biotransformation in LC-MS data. This workflow has been routinely used in our laboratory to support early screening of large numbers of antibody therapeutics. In the case of a molecule with stability liabilities in serum, a continuous decreasing trend of %recovery of intact antibody correlated well with observed catabolites in LC-MS data and/or evidence of aggregation. However, due to the proprietary nature of these molecules, these data cannot be published at this moment.
Acknowledgements: The authors would like to thank our coworker Tyler Curran for his assistance during this work. AbbVie sponsored this study; contributed to the design; participated in collection, analysis, and interpretation of data; and in writing, reviewing, and approval of the final version. Yihan Li and Hetal Sarvaiya are employees of AbbVie and own AbbVie stock.
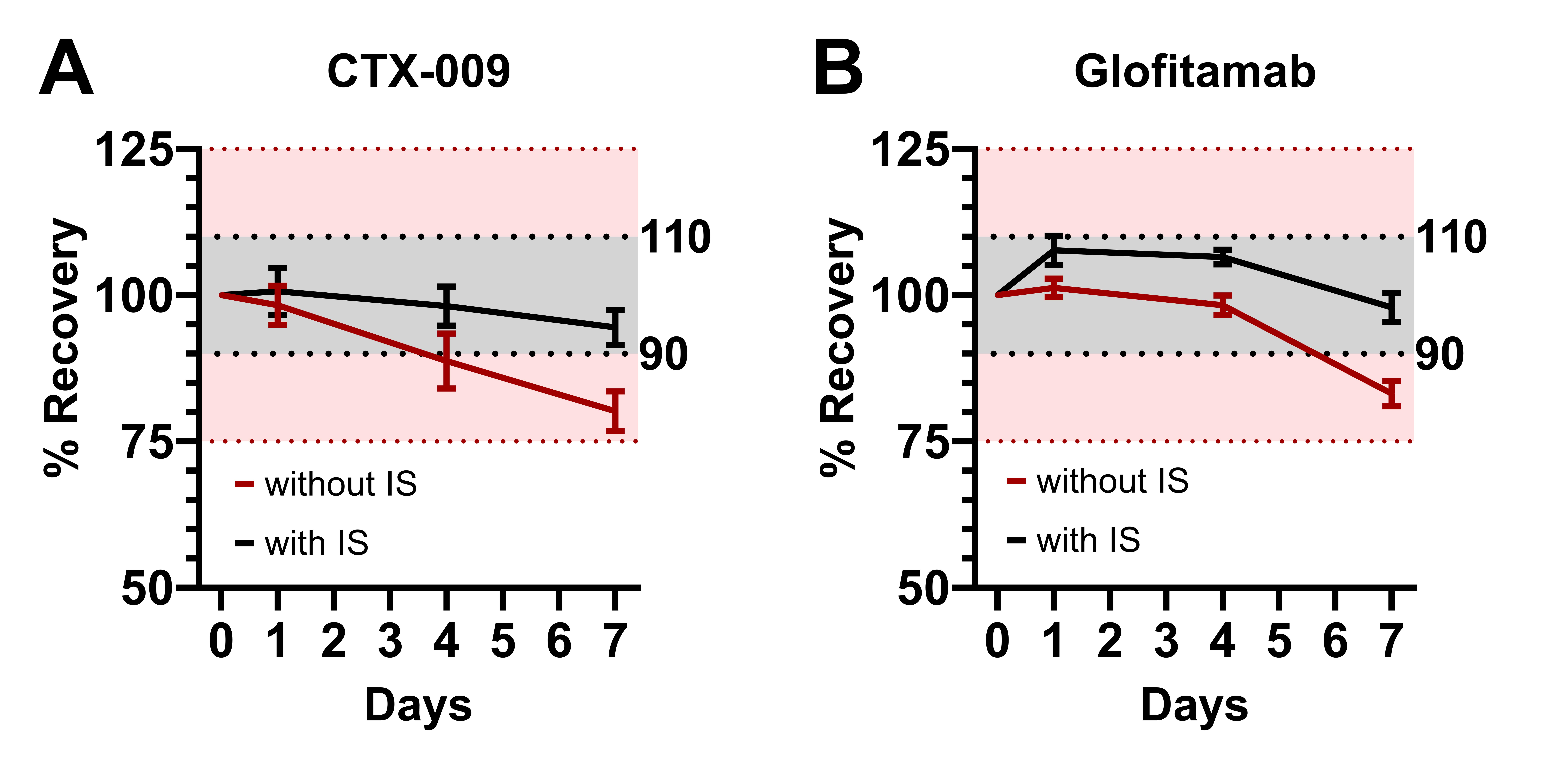 Figure 1. Result comparison of A) CTX-009 and B) Glofitamab, with and without NISTmAb as IS.
Figure 1. Result comparison of A) CTX-009 and B) Glofitamab, with and without NISTmAb as IS.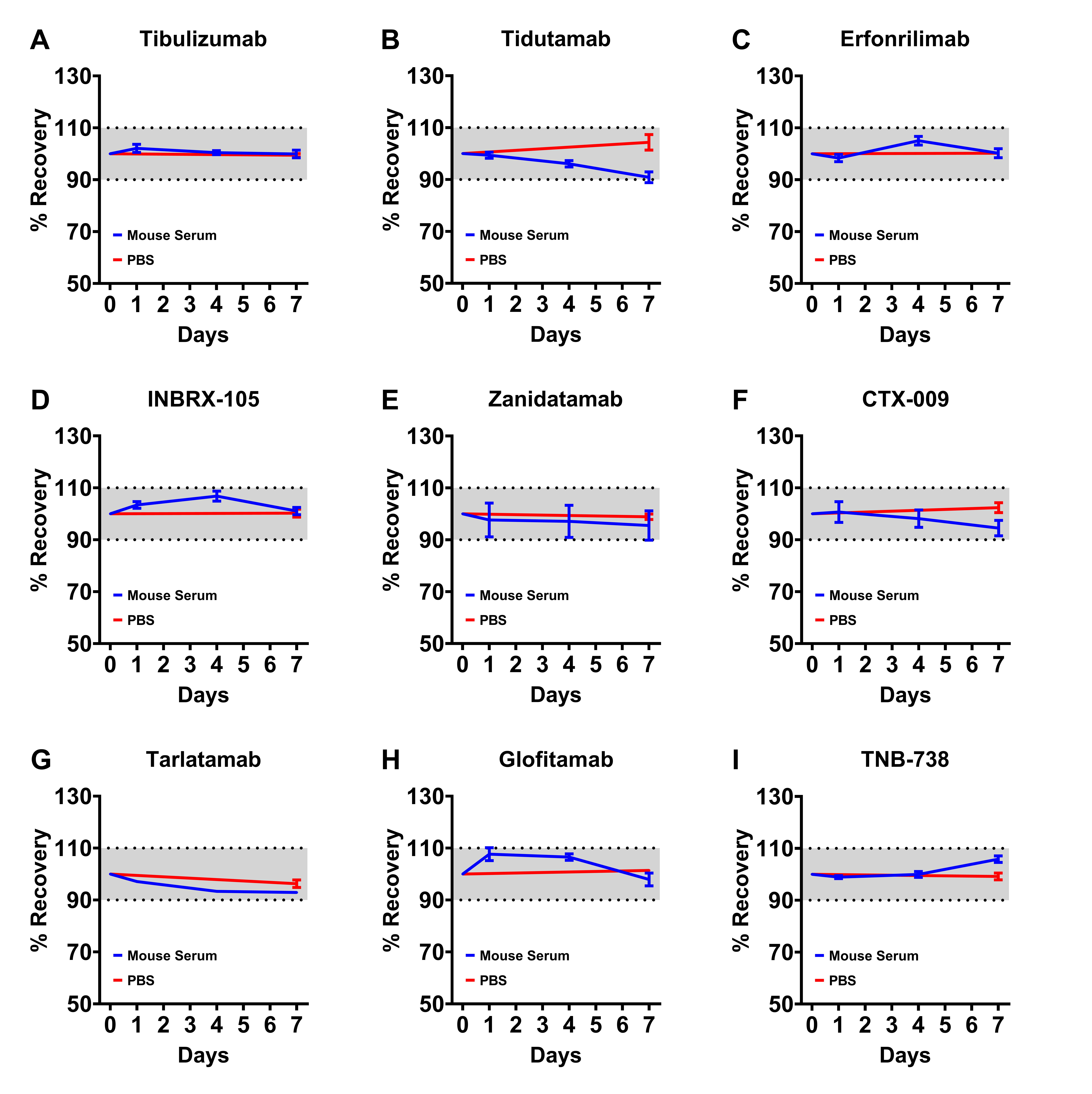 Figure 2. Stability test of 9 molecules in mouse serum with NISTmAb as internal standard: A) Tibulizumab, B) Tidutamab, C) Erfonrilimab, D) INBRX-105, E) Zanidatamab, F) CTX-009, G) Tarlatamab, H) Glofitamab, and I) TNB-738.
Figure 2. Stability test of 9 molecules in mouse serum with NISTmAb as internal standard: A) Tibulizumab, B) Tidutamab, C) Erfonrilimab, D) INBRX-105, E) Zanidatamab, F) CTX-009, G) Tarlatamab, H) Glofitamab, and I) TNB-738.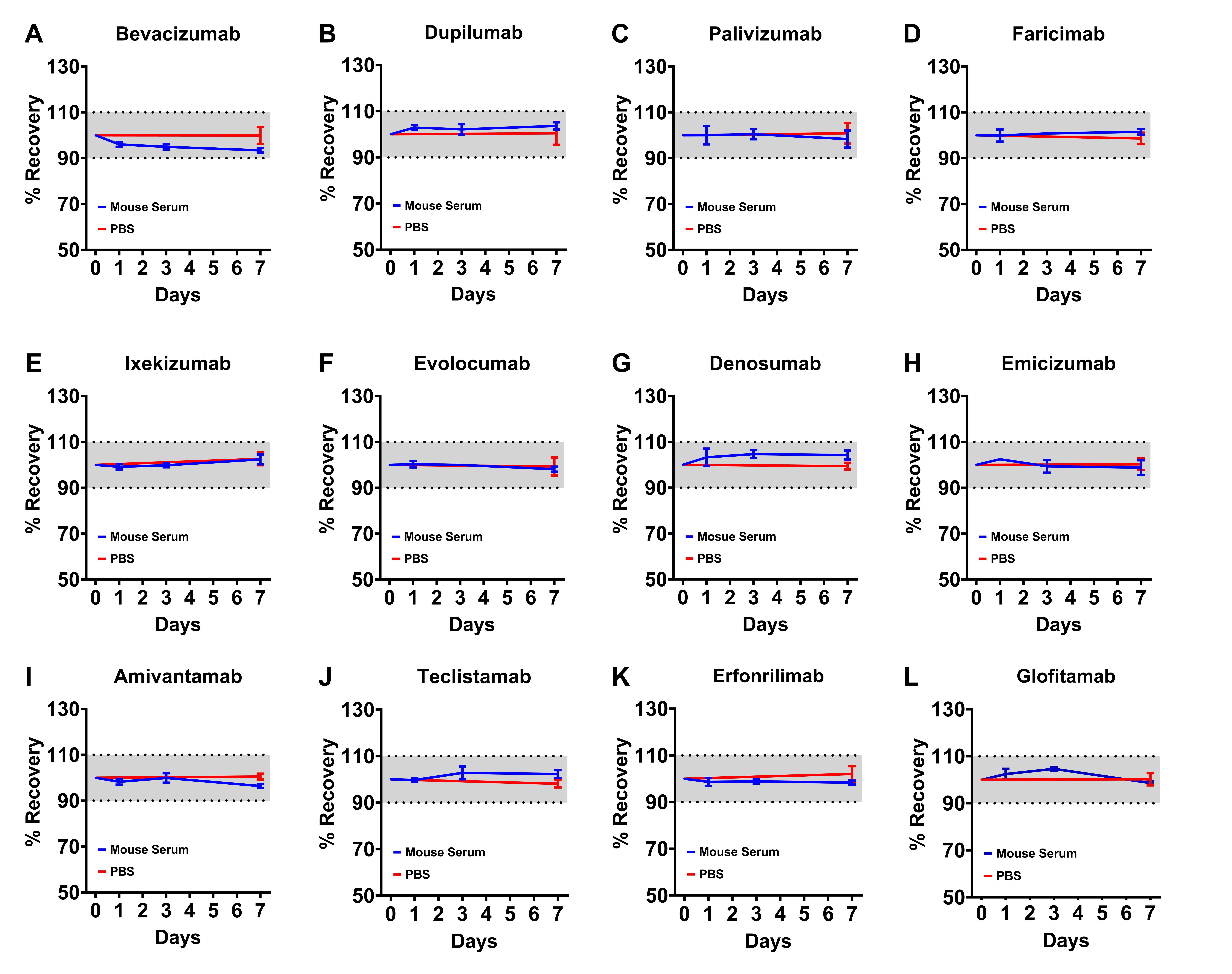 Figure 3. Stability test of 12 molecules in mouse serum with Fc fragment of NISTmAb as internal standard: A) Bevacizumab, B) Dupilumab, C) Palivizumab, D) Faricimab, E) Ixekizumab, F) Evolocumab, G) Denosumab, H) Emicizumab, I) Amivantamab, J) Teclistamab, K) Erfonrilimab, L) Glofitamab.
Figure 3. Stability test of 12 molecules in mouse serum with Fc fragment of NISTmAb as internal standard: A) Bevacizumab, B) Dupilumab, C) Palivizumab, D) Faricimab, E) Ixekizumab, F) Evolocumab, G) Denosumab, H) Emicizumab, I) Amivantamab, J) Teclistamab, K) Erfonrilimab, L) Glofitamab.