Track 2: Novel Strategies to Advance Biotherapeutic Development
Category: Poster Abstract
(T1330-02-07) Optimizing and Validating an In Vitro Transcytosis Assay for Predicting Pharmacokinetics in Drug Discovery and Process Development
Tuesday, May 14, 2024
1:30 PM - 2:30 PM PT

Chang Liu, PhD
Principal Scientist
Genentech, Inc.
South San Francisco, California, United States
Chang Liu, PhD
Principal Scientist
Genentech, Inc.
South San Francisco, California, United States- YW
Yenny Webb Vargas
Genentech, Inc.
South San Francisco, California, United States - SM
Shuxia Meng
Genentech, Inc.
South San Francisco, California, United States - XT
Xuefei Tian
Genentech, Inc.
South San Francisco, California, United States - SS
Steven Swanson
Genentech, Inc.
South San Francisco, California, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Assessment of appropriate pharmacokinetic (PK) characteristics is imperative for the successful advancement of IgG-based biotherapeutics. However, the process can be time-consuming, costly, and reliant on animal studies. A reliable and efficient screening method for PK evaluation is crucial, particularly in early drug discovery to eliminate candidates with undesirable PK properties and in process development to assess the impact of varying conditions. We developed an FcRn-dependent cell-based assay—the transcytosis assay—into a thaw-and-use format to reduce run-to-run variability, optimized various procedures and conditions, and validated the assay using a fit-for-purpose approach to demonstrate consistent and reliable results suitable for applications such as drug screening and process development.
Methods: The transcytosis assay constitutes a two-step methodology involving cell-based transcytosis experiments and subsequent ELISA quantification of transcytosis samples (Chung et al., 2019). A clonal MDCK cell line, genetically modified to express human FcRn, was cultured on transwell plates to establish a polarized monolayer. Test antibodies were introduced into the inner chamber and incubated to facilitate FcRn-mediated transcytosis and subsequent release into the outer chamber growth media. Transcytosis samples were then collected and quantified. For assay optimization, we systematically explored diverse conditions, including the thaw-and-use methodology, variations in cell seeding density and passage numbers, adjustments to cell culture time and antibody incubation duration. For assay validation, we investigated the transcytosis outputs of five monoclonal antibodies (mAbs) exhibiting distinctive human clearance values (Xolair, Actemra, Avastin, mAb1, and mAb2). The validation experimental design comprised 18 transcytosis experiments, testing all five antibodies in eight replicates and measured repeatedly in three independent ELISA runs. The experimental design investigated five key assay factors: two analysts, three batches of cells, two transcytosis plate manufacturers, three transcytosis plate batches per manufacturer, and nine ELISA runs. A separate experiment was performed to assess repeatability, consisting of ten repeats of Actemra samples assayed in one transcytosis run. Various statistical methods were utilized to measure and visualize the performance of the assay.
Results: We minimized the fluctuation of assay outputs by converting a transcytosis assay into a new thaw-and-use format and implementing internal assay controls for data normalization. The performance of the optimized assay was then evaluated using a fit-for-purpose (FFP) validation with a comprehensive experimental design on various assay-contributing factors. The results demonstrated robust performance across the validation parameters. For repeatability, the coefficient of variation (CV) was estimated to be 3.5% (95% Confidence Bound 6.0%). For linearity, transcytosis assay results exhibited a strong positive correlation with human clearance values for the four benchmark molecules (Figure 1; Pearson correlation of 0.969, R2 of 0.939). For intermediate precision, results across the tested monoclonal antibodies (mAbs) showed a maximum CV of 11.1% (maximum 95% Confidence Bound 13.6%). Additionally, variance component analysis revealed differential contributing factors to the variance for fast versus slow clearance molecules, respectively. The optimized method was then applied to case studies involving the PK prediction for a larger group of biotherapeutics or drug candidates, exhibiting statistically strong correlation with their diverse PK behaviors. Furthermore, the method was employed to investigate the impact on PK resulting from process development issues.
Conclusion: The robust validation results from critical analytical and functional performance characteristics of the transcytosis assay, along with case studies demonstrating strong correlation with human PK data, suggest that the optimized assay is adequate to support its intended context of use. This enhances our confidence in utilizing it for evaluating PK during screening biotherapeutic candidates and process development.
References: Chung, S., et al., An in vitro FcRn- dependent transcytosis assay as a screening tool for predictive assessment of nonspecific clearance of antibody therapeutics in humans. MAbs, 2019. 11(5): p. 942-955.
Liu, C., et al., A cell-based FcRn-dependent recycling assay for predictive pharmacokinetic assessment of therapeutic antibodies. Bioanalysis, 2021.
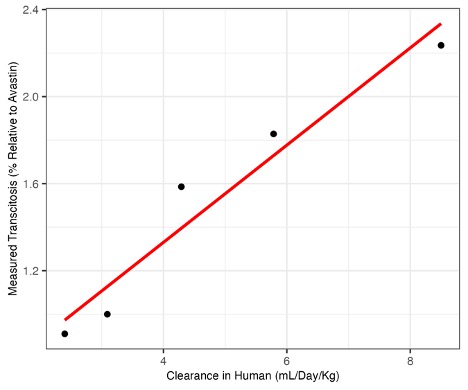 Figure 1. Assay linearity plot. Each data point represents the mean of 43 measured transcytosis results from 43 runs that passed system suitability criteria. The transcytosis assay shows strong linearity when compared to a range of human clearance (Pearson correlation of 0.969, R2 of 0.939)
Figure 1. Assay linearity plot. Each data point represents the mean of 43 measured transcytosis results from 43 runs that passed system suitability criteria. The transcytosis assay shows strong linearity when compared to a range of human clearance (Pearson correlation of 0.969, R2 of 0.939)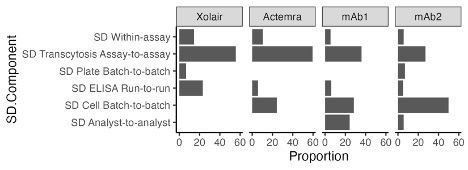 Figure 2. Contributions to the variance of the transcytosis outputs for different mAbs. For mAbs with low transcytosis values, like Xolair, most of the variation in the transcytosis measurement is due to the transcytosis run. For mAbs with higher transcytosis values, like mAb2, the batch of cells used in the transcytosis assay contributes most to the variance, followed by the transcytosis run. Small contributions to the variance came from analyst-to-analyst variation, ELISA run-to-run, transcytosis plate batch-to-batch, and within-transcytosis assay runs.
Figure 2. Contributions to the variance of the transcytosis outputs for different mAbs. For mAbs with low transcytosis values, like Xolair, most of the variation in the transcytosis measurement is due to the transcytosis run. For mAbs with higher transcytosis values, like mAb2, the batch of cells used in the transcytosis assay contributes most to the variance, followed by the transcytosis run. Small contributions to the variance came from analyst-to-analyst variation, ELISA run-to-run, transcytosis plate batch-to-batch, and within-transcytosis assay runs.