Track 2: Novel Strategies to Advance Biotherapeutic Development
Category: Poster Abstract
(T1330-02-06) Utilizing a Bead-Based Sample Pretreatment to Overcome Drug Interference in Developing a Ligand Binding Neutralizing Antibody Assay
Tuesday, May 14, 2024
1:30 PM - 2:30 PM PT
- RM
Rachel Melendez
Genentech, Inc.
South San Francisco, California, United States - RM
Rachel Melendez
Genentech, Inc.
South San Francisco, California, United States - BL
Bob Liu
Genentech, Inc.
South San Francisco, California, United States - AA
Audrey Arjomandi
Genentech, Inc.
South San Francisco, California, United States - ZY
Zhaojun Yin
Genentech, Inc.
South San Francisco, California, United States - WL
Wenyu Liu
Genentech, Inc.
South San Francisco, California, United States 
Kate Peng
Sr Director And Senior Principal Scientist
Genentech, Inc.
South San Francisco, California, United States
Presenting Author(s)
Main Author(s)
Co-Author(s)
Purpose: Assessing neutralizing antibody (NAb) activity is critical for understanding the overall immunogenicity for biological therapeutics. Here, we describe the development of a NAb assay to support a monoclonal antibody program in clinical studies. The fully human monoclonal antibody competitively inhibits receptor function. The challenge for this assay is to be able to detect NAb activities with the desired sensitivity in the presence of high circulating drug in patient serum samples. Current clinical studies are evaluating a dosing regimen with a Ctrough drug concentration expected up to ~250 μg/mL. Here, we used bead-based sample pretreatment to overcome high drug interference and fulfilled the drug tolerance requirement in a ligand binding NAb assay format.
Methods: Sample pretreatment utilizes glycine acidic pH buffer to dissociate drug:anti-drug antibody (ADA) complexes, followed by neutralization with a solution containing biotinylated drug to capture the ADA using streptavidin-coated magnetic beads. Captured ADA was re-dissociated with a second glycine acidic pH buffer, and biotinylated drug with streptavidin-coated beads were removed. The final sample containing ADA was neutralized and subjected to ligand binding format described in Figure 1.
Results: The main consideration for the assay development was overcoming drug tolerance greater than 250 μg/mL of monoclonal antibody drug while still being able to detect 1000 ng/mL of ADA. A number of different assay parameters and methods were tested. Cell-based format was first explored with various sample pretreatment methods including Precipitation, Acid Dissociation and Biotin-Drug as Assay Drug (PABAD) and removing excess drug with biotinylated target; but unfortunately, did not have sufficient drug tolerance. We then pursued a competitive ligand binding assay (CLBA) format since the drug is an antagonist that competitively blocks receptor function. Using this CLBA assay format together with beads based sample pretreatment, we were able to achieve sensitivity of approximately 300 ng/mL of the surrogate positive control antibody (polyclonal anti-ID against the monoclonal antibody drug), and the assay was able to detect 1000 ng/mL of the surrogate positive control in the presence of 300 μg/mL of monoclonal antibody drug (Table 1).
Conclusion: Drug interference was presented as a major challenge during clinical NAb assay development. Both cell- based and ligand binding formats were evaluated. Ultimately using a combination of bead-based sample pretreatment and the ligand binding format, we were able to successfully overcome the drug interference issue and delivered a sensitive and drug tolerant NAb assay.
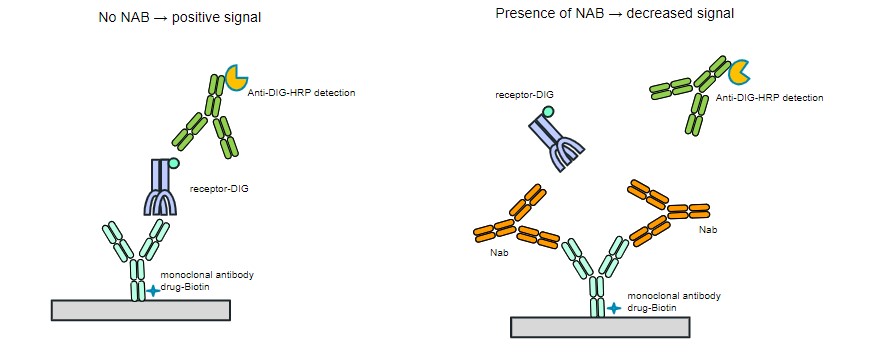 Figure 1. Post-treatment samples were incubated onto a streptavidin coated plate that was preloaded with biotinylated drug. After overnight incubation, the plate was washed, and digoxin-labeled soluble target added to test for neutralizing activity. After washing, an anti-digoxin HRP conjugate was used for detection followed by colorimetric development using TMB substrates. If no NAb were present, the signal was expected to be high (left panel), whereas in the presence of NAb, the signal was expected to be low (right panel).
Figure 1. Post-treatment samples were incubated onto a streptavidin coated plate that was preloaded with biotinylated drug. After overnight incubation, the plate was washed, and digoxin-labeled soluble target added to test for neutralizing activity. After washing, an anti-digoxin HRP conjugate was used for detection followed by colorimetric development using TMB substrates. If no NAb were present, the signal was expected to be high (left panel), whereas in the presence of NAb, the signal was expected to be low (right panel).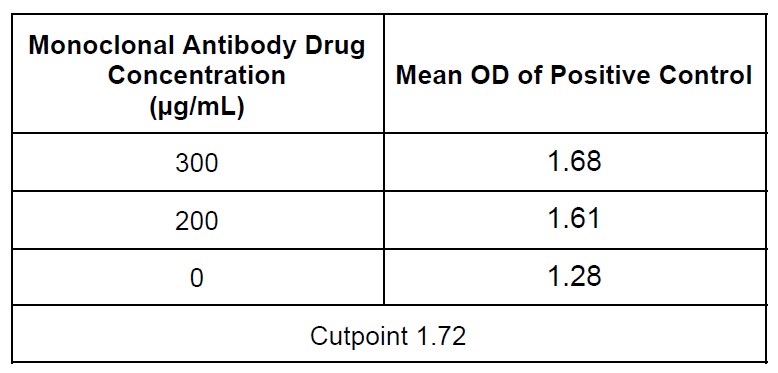 Table 1: Achieving drug tolerance with 1000 ng/mL positive control antibody. A sample producing an assay signal below the cutpoint based on the negative control (normal human serum) would be NAb positive, whereas a signal above the cutpoint would be NAb negative.
Table 1: Achieving drug tolerance with 1000 ng/mL positive control antibody. A sample producing an assay signal below the cutpoint based on the negative control (normal human serum) would be NAb positive, whereas a signal above the cutpoint would be NAb negative.